[Thomson] existed as scientist and technologist, academic and entrepreneur, a philosopher and a practical man rolled into one… [He] had been described in his lifetime as Britain’s and perhaps the world’s greatest scientist. – David Lindley [1]
Thermodynamics developed rapidly in the early 1850s due largely to the rapid back-and-forth progress achieved between Rudolf Clausius and William Thomson. It was Thomson who initially helped set the course Clausius followed. It was then Clausius who surpassed Thomson and inspired him to catch up. It was then Thomson who indeed did catch up and perhaps surpassed Clausius by becoming the leading force behind the rise of energy as a unifying theory in the thermodynamic movement in North Britain. Then it was Clausius who launched the concept of entropy. All told, their collective efforts opened the door to an entirely new realm of science based on energy and entropy. Each fed off the other, which makes this a good segue from my previous post on Clausius to this one on Thomson.
Thomson’s early education was influenced by French thought – heat is coserved
Thomson immersed himself in French science, starting with his enthusiastic devouring of Fourier’s work on heat transfer within two weeks as a 16-year-old and continuing with his time spent studying experimental techniques under Henri Victor Regnault in France. To the French at that time, heat was conserved and Thomson came to accept this belief. But then James Joule came along in 1847 with experimental evidence that heat was NOT conserved, to which Thomson wrote to his brother, if this were indeed true, it would “overturn the whole theory of heat” [2] and necessitate a re-interpretation of all existing data within a new framework, a task too overwhelming to consider. How ironic then that this is exactly what happened, with Thomson leading the way.
The Thomson brothers discover Carnot and are confronted by conduction
Upon reading Sadi Carnot’s Reflections on the Motive Power of Fire, Thomson and his brother James, an established engineer and academic physicist in his own right, were intrigued by the consequences. In 1844 James speculated that it might be possible to operate steam engines without fuel “by using over again the heat which is thrown out in the hot water from the condenser.” [3] And then in 1849 William wrote,
“When “thermal agency” is thus spent in conducting heat through a solid, what becomes of the mechanical effect which it might produce? Nothing can be lost in the operations of nature – no energy can be destroyed. What effect then is produced in place of the mechanical effect which is lost? We may hope that the very perplexing question in the theory of heat, by which we are at present arrested, will, before long, be cleared up.” [4]
The challenge in front of the Thomson brothers was conduction. They couldn’t reconcile thermal conduction with the conservation of heat. In their minds, heat flows into the hot end of an iron bar and out through the cold end. They wondered why the heat exiting into the cold sink couldn’t be re-used.
The science of conduction – there is no such thing as “heat flow”
Do you think that conduction is easy to grasp? Think again. Even after William Thomson’s breakthrough to the mechanical theory of heat in 1851, did he truly understand conduction? I’m not sure. A note from James Clerk Maxwell to William in 1855, years after the launch of energy, is very telling. Wrote Maxwell, “By the way do you profess to account for what becomes of the vis viva of heat when it passes through a conductor from hot to cold? You must either modify Fourier’s laws or give up your theory, at least so it seems to me.”[5] Such questioning revealed a continuing large gap in knowledge regarding energy in the years after its launch in 1850-1.
What was it about conduction that was so difficult to grasp? The answer, as Maxwell’s question suggests, was that heat was still thought of as a flowing entity. Even today, many are confused about this because we still use the words, heat flow. The fact is that there is no flow of heat. There is no entity called heat.
Heat quantifies a change in state
Heat is nothing more than the change in state of a system that results from the exchange of thermal energy across a stationary boundary. The change results from the direct collisions between atoms. For example, in the first step of Carnot’s cycle, molecules from a hot source (combustion products) smash into the external metal wall of a cylinder and cause the cylinder’s metal atoms to vibrate faster around their fixed positions, thereby striking the gas molecules inside the cylinder with higher kinetic energy. As the gas molecules move faster, both temperature and pressure increase and the piston gets pushed out to accomplish work. In analyzing this, we say that heat “leaves” the hot source and “enters” the piston. But this isn’t correct. In reality, the energy of the hot source decreases while the energy of the gas inside the cylinder increases by exactly the same amount, as the conservation of energy is applied to the collisions of the zillions of atoms and molecules involved. The changes are quantified by a term called “heat”, as opposed to similar changes that result when the boundary is moving rather than stationary, which we call “work.” They’re both caused by the same phenomenon: the collision of atoms at interfaces. In this discussion, it doesn’t confuse us too much to refer to heat as leaving or entering. We’re used to this terminology. But when we turn to the process of conduction, confusion appears.
Put a metal bar between a hot source and a cold sink (Figure 32.2 below from my book). The bar provides a means for heat to figuratively drain out of the hot and into the cold, bypassing the engine, thus resulting in a decrease in energy of the former and an increase in energy of the latter by the same amount. (Note: the changes in entropy do not equal each other with conduction.) The magnitude of each change we quantify as “heat.” But then we say that heat “leaves” the hot, “flows” down the bar and “enters” the cold. Here we meet the source of our confusion, because nothing, in fact, flows. In the world comprised of a flowing and conserved heat, before energy was discovered and the atomic theory of matter became known, it was difficult to grasp conduction in the mid-1800s.
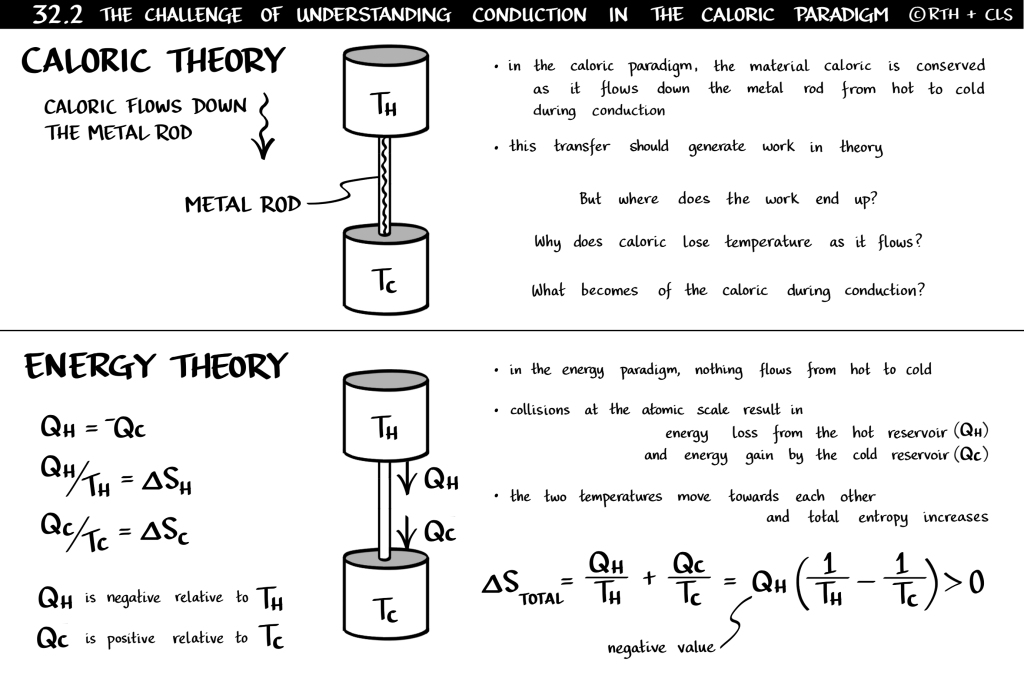
Challenge yourself on what you really know about conduction.
When we look at the steady-state conduction of heat down a metal bar from high temperature to low, we assume that the heat leaving the high equals the heat entering the low since there’s no accumulation (no change in temperature of the bar over time). We refer to this heat as a flow from a high to a low “elevation” which we call temperature. The analogy here, created by Carnot and enthusiastically embraced by the Thomsons, who had separately shown keen interest in water-wheel power generation, was indeed the water-wheel in which high elevation water flowed into the water-wheel buckets, turning the shaft as they fell to lower elevation. It was easy back then to understand this from an “mg∆h” argument. But when you look at conduction, the analogy breaks down. You’re left asking, what happens when heat flows from high temperature to low? Something must be happening, right? Temperature decreases. But does this mean that energy also decreases? But this can’t be since the energy leaving the hot must equal the energy entering the cold. And this is the exact paradigm that Thomson was stuck in, all because of this mental model of a “flowing heat.”
Again, our use of such out-of-date terms and concepts really makes our lives difficult. There is no flow. There is no temperature of heat. There is no entity called heat. It’s an incorrect mechanical model that, while sufficient to guide Fourier’s math, was insufficient to guide Thomson’s understanding of heat and work together in the same context. He couldn’t understand how a flowing heat could change its temperature and cause work in Carnot’s engine but cause nothing in conduction. How confusing this must have been for both brothers. Life was fine when only dealing with conduction. But with the conflicting arrival of Carnot’s heat engine and Joule’s experiments, life was no longer fine.
Even though William may not have fully understood conduction at the time of Maxwell’s note, which may have contributed to his struggle with the draft manuscript of his 1851 Dynamical Theory[6], the need to bring new and exact definitions to the new world of energy became clear to him. When it did, Thomson eventually and carefully changed his use of the word “heat,” ceasing to speak of the heat in a body, “referring only to heat absorbed or emitted with respect to the surroundings, and of ‘thermal motions’ inside.”[7] But even with this new definition, I’m not sure that Thomson understood the distinction between heat and energy.
And to answer the Thomson brothers’ question, if such a short-circuit existed to cause thermal energy to exchange between the hot reservoir and the cold reservoir, the lost opportunity to do work indeed ends up in the cold reservoir in the form of an increase in thermal kinetic energy. But you’ll likely never see this. Why? Because the mass of the cold reservoir is typically quite large, leaving the resulting temperature rise infinitesimal.
Learn more, much much more, about the Thomson brothers!
Learn more about the William Thomson and his brother James in Chapter 32 of my book, Block by Block – The Historical and Theoretical Foundations of Thermodynamics. You might be very interested in how James used Carnot’s work to (successfully) hypothesize the impact of pressure on the melting temperature of ice!
References
[1] Lindley, David, 2004. Degrees Kelvin: A Tale of Genius, Invention, and Tragedy. Washington, D.C: Joseph Henry Press, pp. 4-5.
[2] Cardwell, D. S. L. 1971. From Watt to Clausius; the Rise of Thermodynamics in the Early Industrial Age. Ithaca, N.Y: Cornell University Press., p.244.
[3] Smith, Crosbie. 1998. The Science of Energy: A Cultural History of Energy Physics in Victorian Britain. Chicago: University of Chicago Press., p. 42.
[4] Thomson quote cited in Smith, Crosbie. 1998. The Science of Energy: A Cultural History of Energy Physics in Victorian Britain. Chicago: University of Chicago Press, p. 94.
[5] Smith, Crosbie. 1998. The Science of Energy: A Cultural History of Energy Physics in Victorian Britain. Chicago: University of Chicago Press, p. 247.
[6] Smith, Crosbie. 1998. The Science of Energy: A Cultural History of Energy Physics in Victorian Britain. Chicago: University of Chicago Press, p. 101.
[7] Smith, Crosbie W., and Matthew Norton Wise. 2009. Energy and Empire: A Biographical Study of Lord Kelvin. Cambridge: Cambridge Univ. Press, p. 338.
END


Leave a comment